National Service Hotline:027-63495513China


The circulating water has sufficient oxygen, suitable temperature and eutrophic conditions, which is very suitable for the growth and reproduction of microorganisms and bacteria and algae. If not controlled in time, the water quality will rapidly deteriorate, odor, black, a large number of deposits and even clogging equipment and piping, not only affecting the condenser and the use of the heat transfer system, as well as potential safety hazards. Therefore, circulating water treatment must control microorganisms and algae reproduction.
Commonly used disinfectants today are sodium hypochlorite, chlorine gas, chlorine dioxide and ozone.
Sodium hypochlorite, chemical formula NaClO, is the hypochlorite of sodium. It is a highly effective chlorine disinfectant, and oxidation is its main bactericidal mechanism. Sodium hypochlorite hydrolyzed to form hypochlorous acid, hypochlorous acid further decomposition to form the new ecological oxygen, the new ecological oxygen of the strong oxidizing bacteria and viruses on the protein and other substances denatured, so as to cause death of pathogenic microorganisms. In addition, hypochlorous acid in the process of sterilization, virus killing, not only can act on the cell wall, virus shell, and because hypochlorous acid molecules are small, uncharged, but also penetrate into the bacteria (virus) body, and bacteria (virus) body proteins, nucleic acids, and enzymes and other organic macromolecules undergo oxidation reaction, thus killing the pathogenic microorganisms. At the same time, hypochlorous acid produces chlorine ions can also significantly change the osmotic pressure of bacteria and viruses, so that their cell inactivity and death.
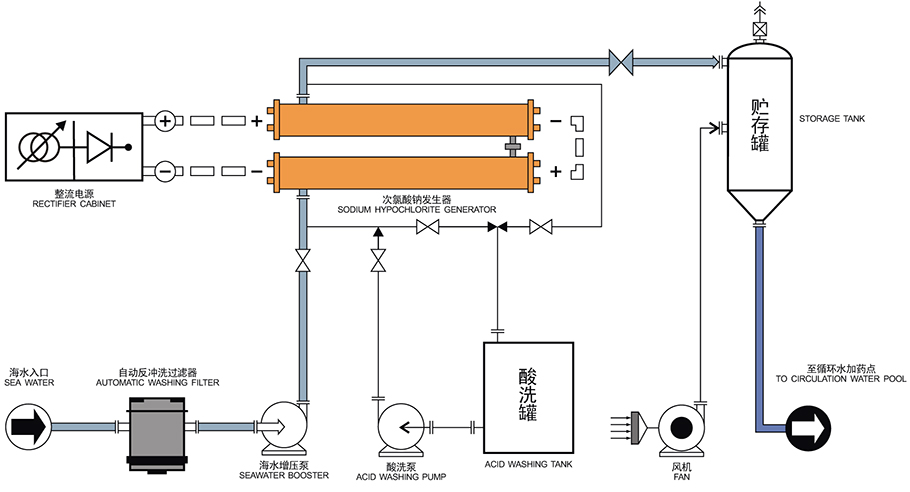
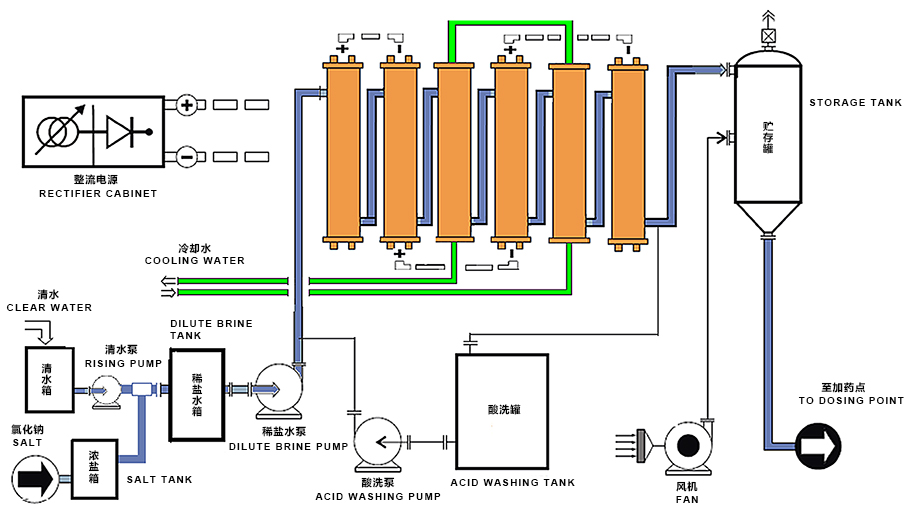
Sodium hypochlorite solution by electrolyzing is efficient, safe, economical, non-polluting, and is the first choice for industrial circulating water disinfection and sterilization.
Good disinfection
Based on chemical measurements, PPM-level concentrations of sodium hypochlorite are almost completely hydrolyzed to hypochlorous acid in water at efficiencies greater than 99.9%.
High security
Sodium hypochlorite solution by electrolyzing is produced and used on-site, which is relatively safe and does not have the safety hazards of leakage of chlorine gas, chlorine dioxide and other gaseous disinfectants.
Good economics
Sodium hypochlorite solution by electrolyzing is relatively inexpensive to produce, especially when derived from widely available and inexpensive industrial salt or seawater. At the same time, sodium hypochlorite solution, being inter-dissolved in water, can be dosed with precise control, avoiding wastage.
Non-polluting to the environment
Excess sodium hypochlorite will naturally decompose into oxygen and sodium chloride, which are non-polluting to the environment and will not form secondary pollution.